Lymphatic filariasis
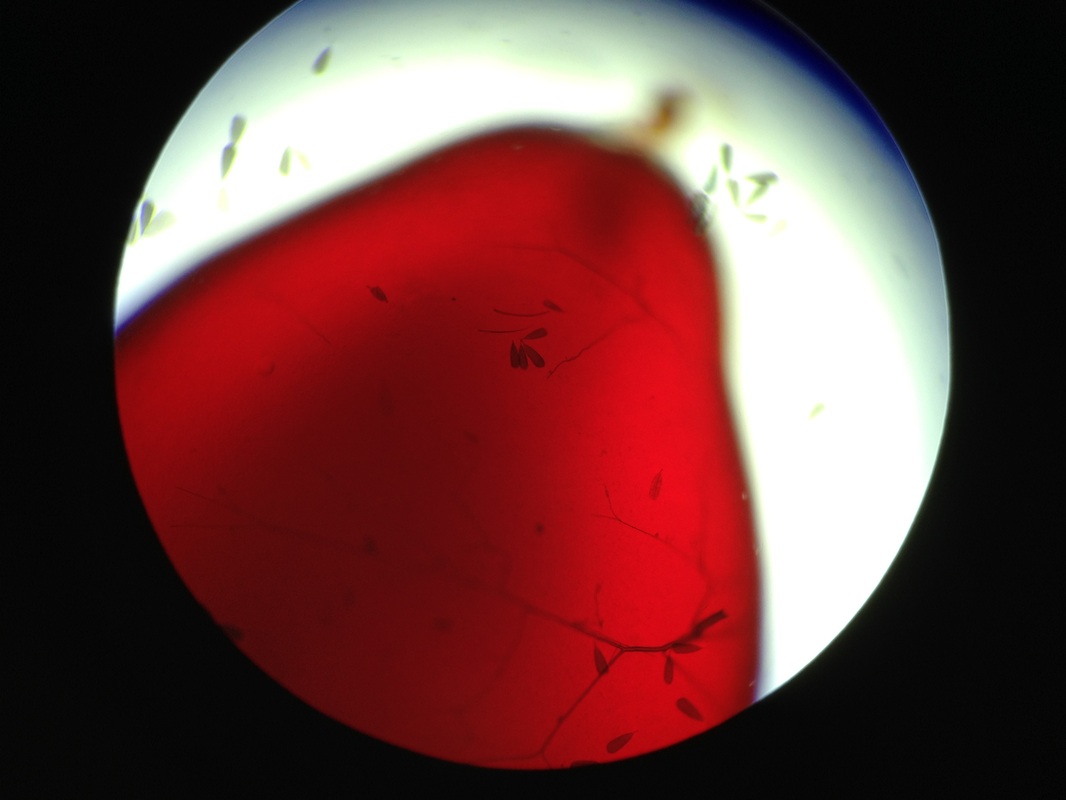
 Intertwined Brugia adults. Taken by my iPhone.
Intertwined Brugia adults. Taken by my iPhone.
Lymphatic filariasis (LF) is a neglected tropical disease caused by the parasitic nematodes Brugia malayi, Brugia timori, and Wucheria bancrofti and has been targeted by the World Health Organization for eradication by 2020. Nearly 1.4 billion people in 73 countries are at risk of infection and 120 million are currently infected, with 40 million people disfigured and debilitated by the disease. Also known as elephantiasis, infection with these nematodes causes alteration to the lymphatic system resulting in fever, lymphadenitis, lymphangitis, lymphedema, and secondary bacterial infection.
A challenge in working with parasitic nematodes is the complexity of their lifecycles and the difficulty in maintaining these organisms in a lab environment. Wucheria bancrofti causes 90% of LF cases, but cannot be maintained in a lab. Brugia malayi, which causes most of the remaining cases, can be propagated in a laboratory through Aedes mosquitoes and a mammalian host, often jirds. Brugia species have four larval stages, termed L1-L4, with molts occurring between each transition and a final pubescent molt when L4 animals transition to adulthood.
A challenge in working with parasitic nematodes is the complexity of their lifecycles and the difficulty in maintaining these organisms in a lab environment. Wucheria bancrofti causes 90% of LF cases, but cannot be maintained in a lab. Brugia malayi, which causes most of the remaining cases, can be propagated in a laboratory through Aedes mosquitoes and a mammalian host, often jirds. Brugia species have four larval stages, termed L1-L4, with molts occurring between each transition and a final pubescent molt when L4 animals transition to adulthood.
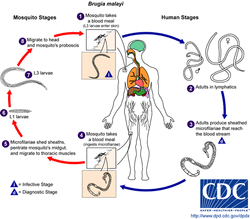 Courtesy of CDC website
Courtesy of CDC website
The B. malayi lifecycle begins with infected mosquitoes biting human hosts. L3 larvae, residing in the mosquitoes “lower lip” or labrum, enter the puncture wound and enter the blood stream. The larvae molt twice to become adults and following mating, females produce specialized sheathed eggs (microfilaririae); females can live for an average of 6-8 years and produce 10,000 mfs a day. These mfs must sense cues in the host and migrate to the peripheral capilleries at night, the time when mosquitoes are most active. Once a mosquito takes an infected blood meal the mfs lose their sheath to become L1 larvae, which then penetrate the mosquito midgut and migrate to the thoracic muscle, where the L2 molt happens. Finally, the L2 animals make their way to the head of the mosquito where the L3 larvae develop and enter the labrum to continue the infection cycle.
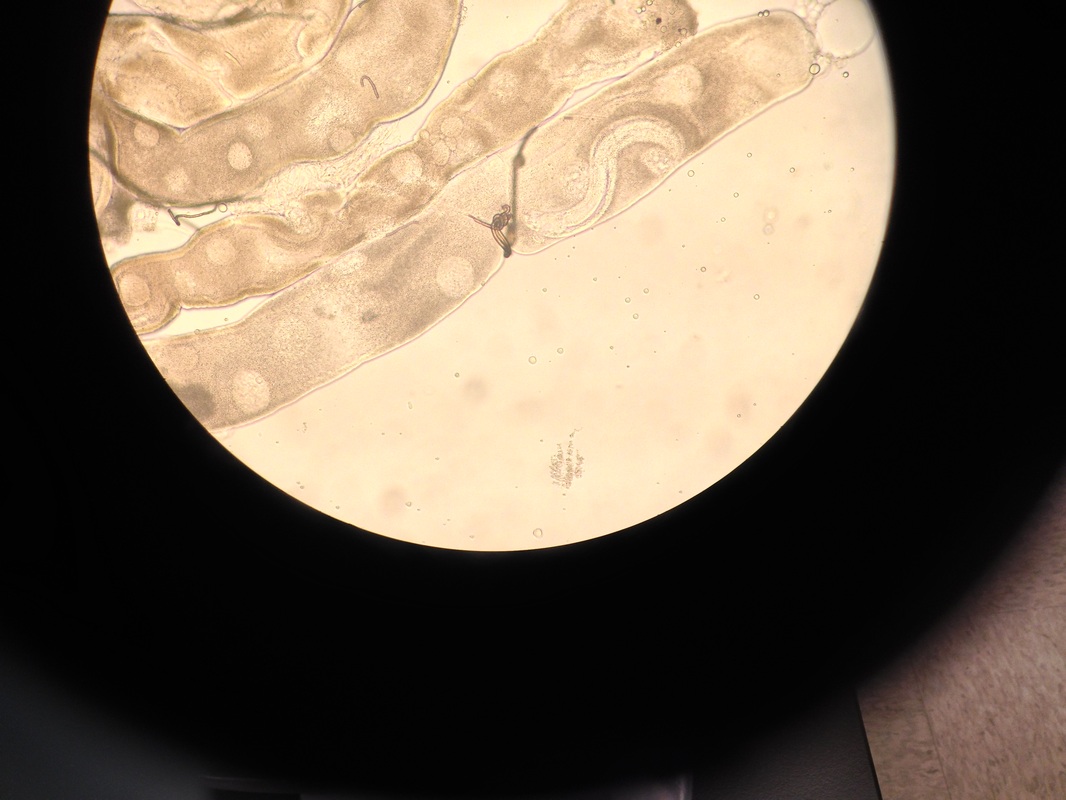
 Culturing Aedes aegypti mosquito larvae.
Culturing Aedes aegypti mosquito larvae.
This lifecycle highlights many of the fascinating and important aspects of host-parasite dynamics. The parasite is finely attuned to host physiology, sensing circadian cues in order to migrate to appropriate tissues to continue the infection cycle. Parasites are often intimately dependent on specific hosts. For example, the dog heartworm Dirofilaria imminitis, a close relative of Brugia, can infect humans and migrate to the lungs as it would during infection of its canine host. However, the parasite can develop no further and the worm dies, leading to a granuloma that is frequently mistaken for lung cancer on X-ray (see wikipedia page). Another aspect of the host-parasite relationship of particular relevance to my work in the tissue specificity of the molts. Brugia molts take place in specific times and places in the mosquito host; when and where they molt in mammalian hosts is less clear. Dirofilaria imminitis, undergoes its L2 molt in a completely distinct tissue than B. malayi, the Malpighian tubules, which is the mosquito equivalent of the kidney. It seems almost certain that the parasite is sensing host cues to coordinate these developmental transitions, but the nature of these cues is elusive. Nuclear hormone receptors sample signaling molecules and metabolites in virtually all taxa and control molting in arthropods, and are thus perfectly positioned to regulate these molts. As nuclear hormone receptors are a “druggable” protein, they are an attractive candidate for therapeutic intervention. Yet, I strongly feel that we must uncover the basic biology of how nuclear receptors regulate Brugia malayi molting in order to fully realize their potential as a drug target.
Links:
- Centers for Disease Control Lymphatic Filiriasis site
- World Health Organization Lymphatic Filiriasis site
- Wikipedia Brugia malayi site
- Video of L3 Brugia pahangi emerging from a mosquito labrum
- Pictures of the infection process. Taken by Dr. Steven Lindsay who infected himself with Brugia pahangi in 1982 and documented the infection process (scroll halfway down page)